The aim of organic synthesis is the construction of more complex molecules from simpler structures. This process involves the chaining of reactions from a given substrate to obtain the molecule that is the object of the synthesis.

In molecules with several functional groups, synthetic procedures become, practically in a “tailor-made suit”for each case, since there are so many types of reactions and ways to obtain a molecule (functional group), that the adaptation of the reactions of organic chemistry to multifunctional compounds is a hard work, in which creativity and intuition have a prominent role. Therefore, it is not surprising that some authors speak of organic synthesis in terms of art.
In basic courses of Organic Chemistry, organic synthesis is based on the use of the reactions that are learned with the development of the programs, for the preparation, in a few steps, of molecules with a limited number of functional groups and with carbon skeletons that are not too large.
Despite these relatively modest objectives, students encounter serious difficulties in tackling these exercises, since it is necessary to relate multiple concepts and acquire a global vision of the knowledge acquired.
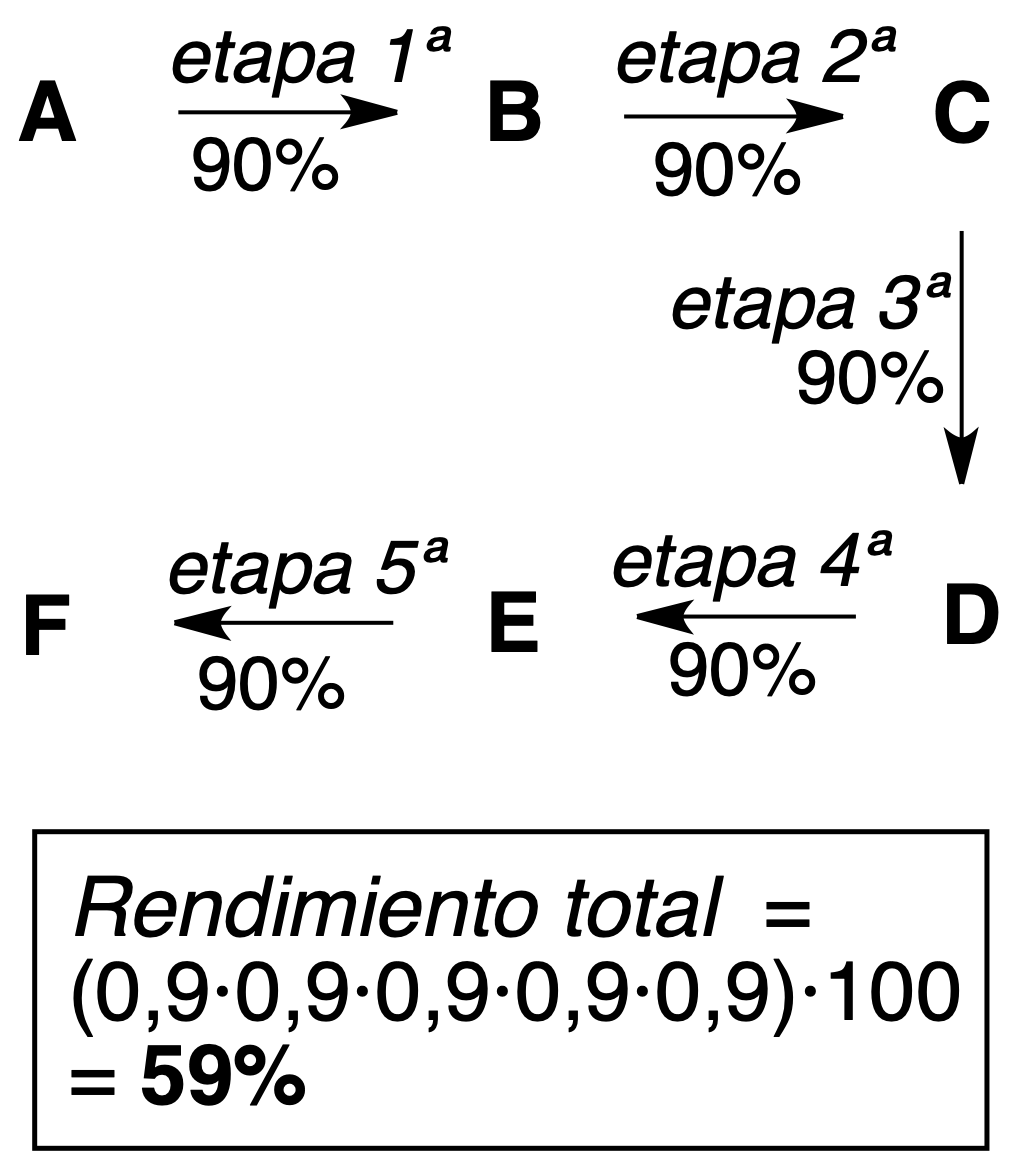
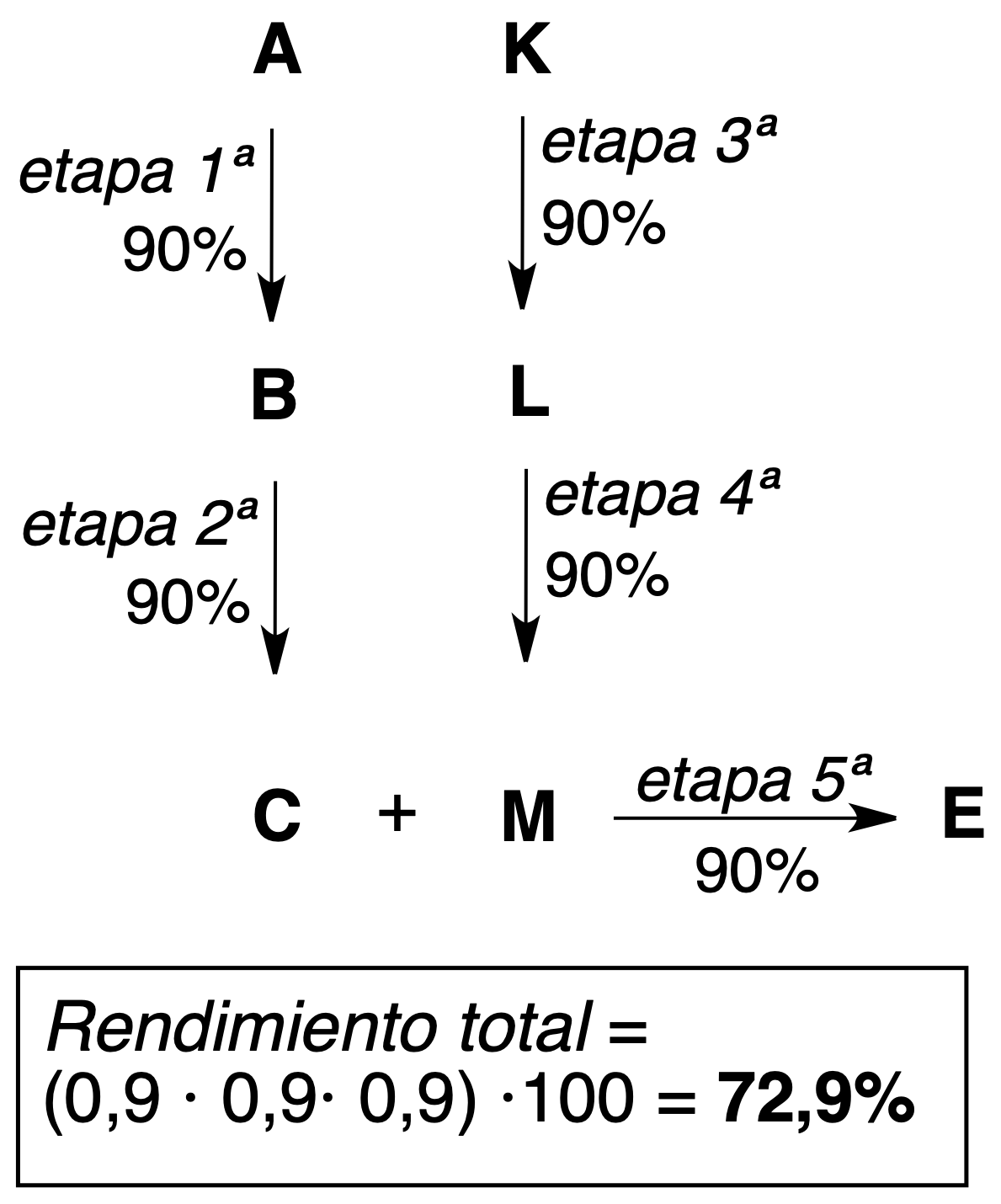
As seen in the above examples of 5-step processes, higher theoretical yields (more efficient) are obtained with convergent synthesis.
Useful concepts to classify reagents and reactions:
From another point of view, the types of reactions that are used when a given organic synthesis is considered can be grouped into two main categories, which are discussed in the following two sections.
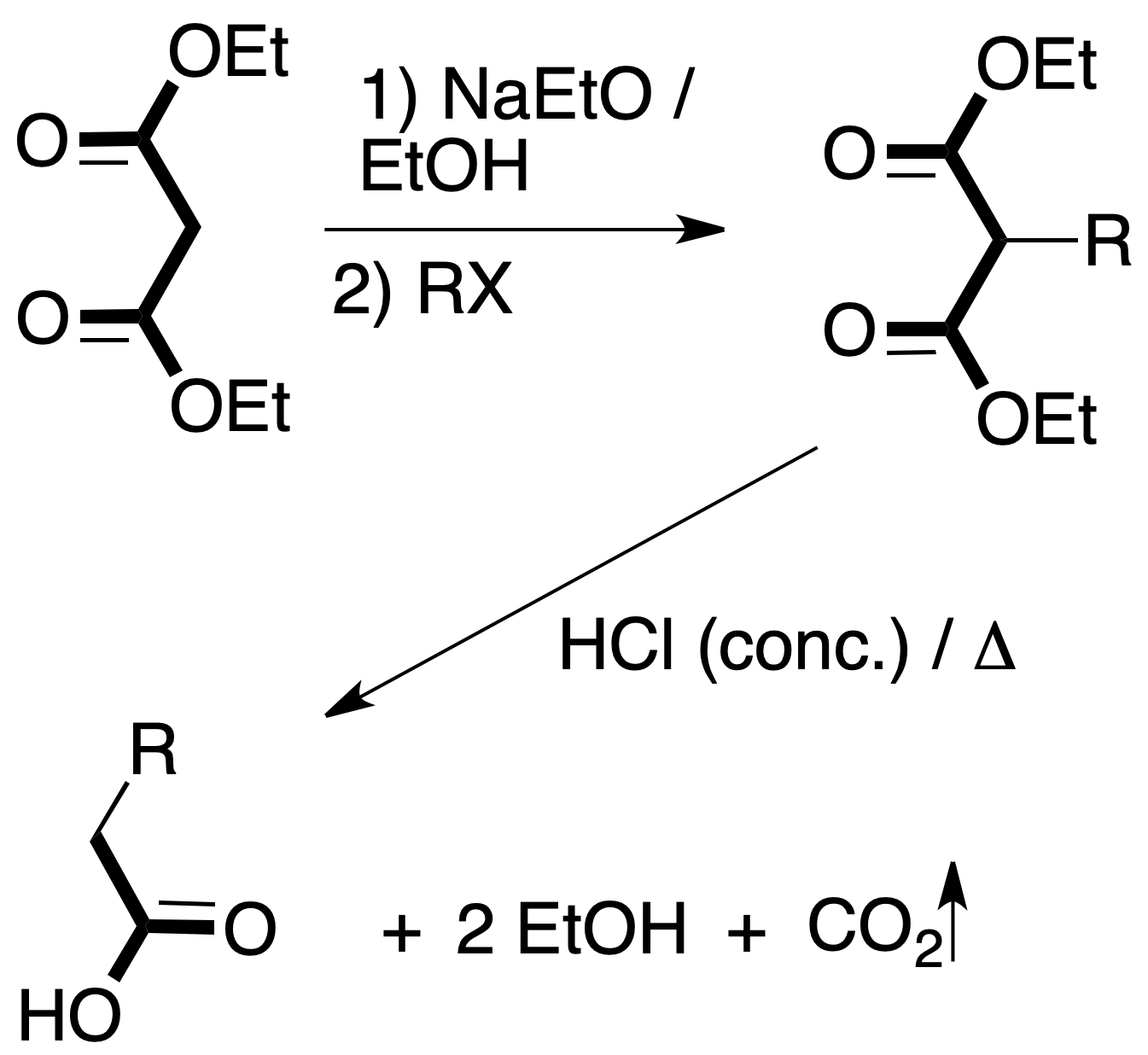
Within organic synthesis, an important section is the FGI. In molecules with a single function, this task is not a great difficulty, since it is sufficient to have a basic knowledge of the chemistry of functional groups.
If the next molecule is treated with a reducing agent, both the ester function and the ketone function are susceptible to reduction.
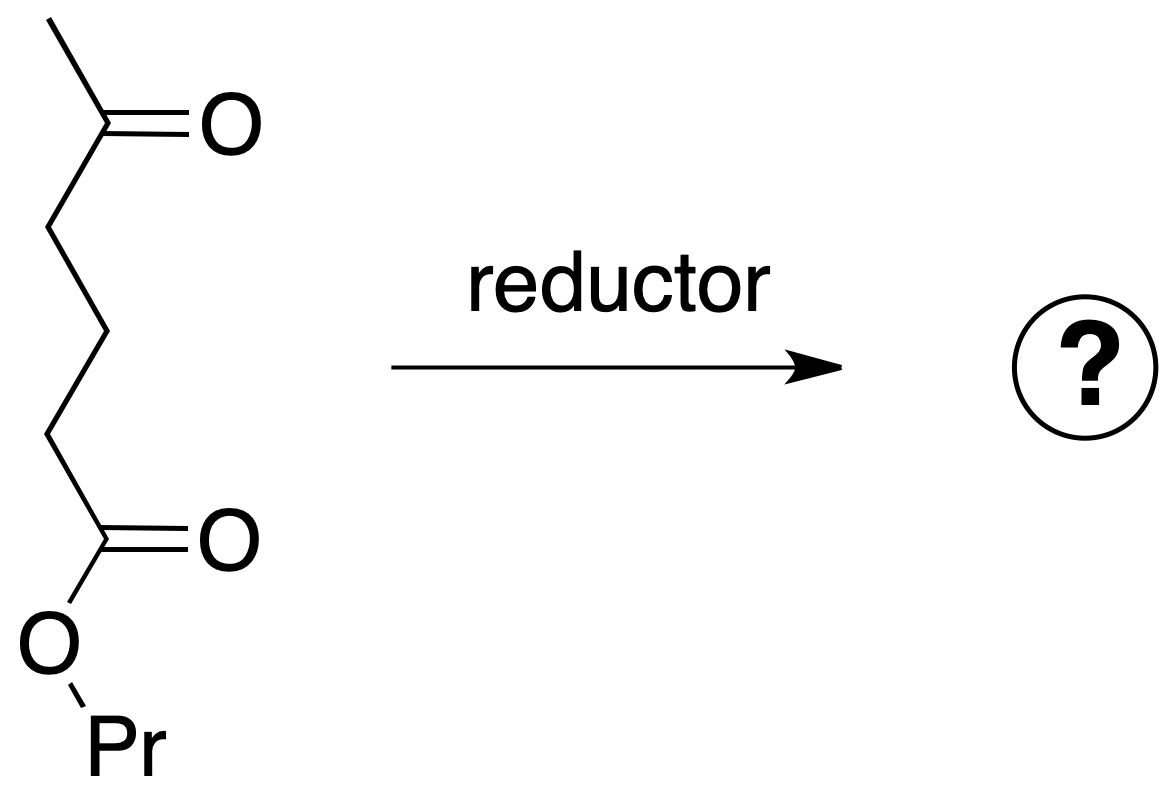
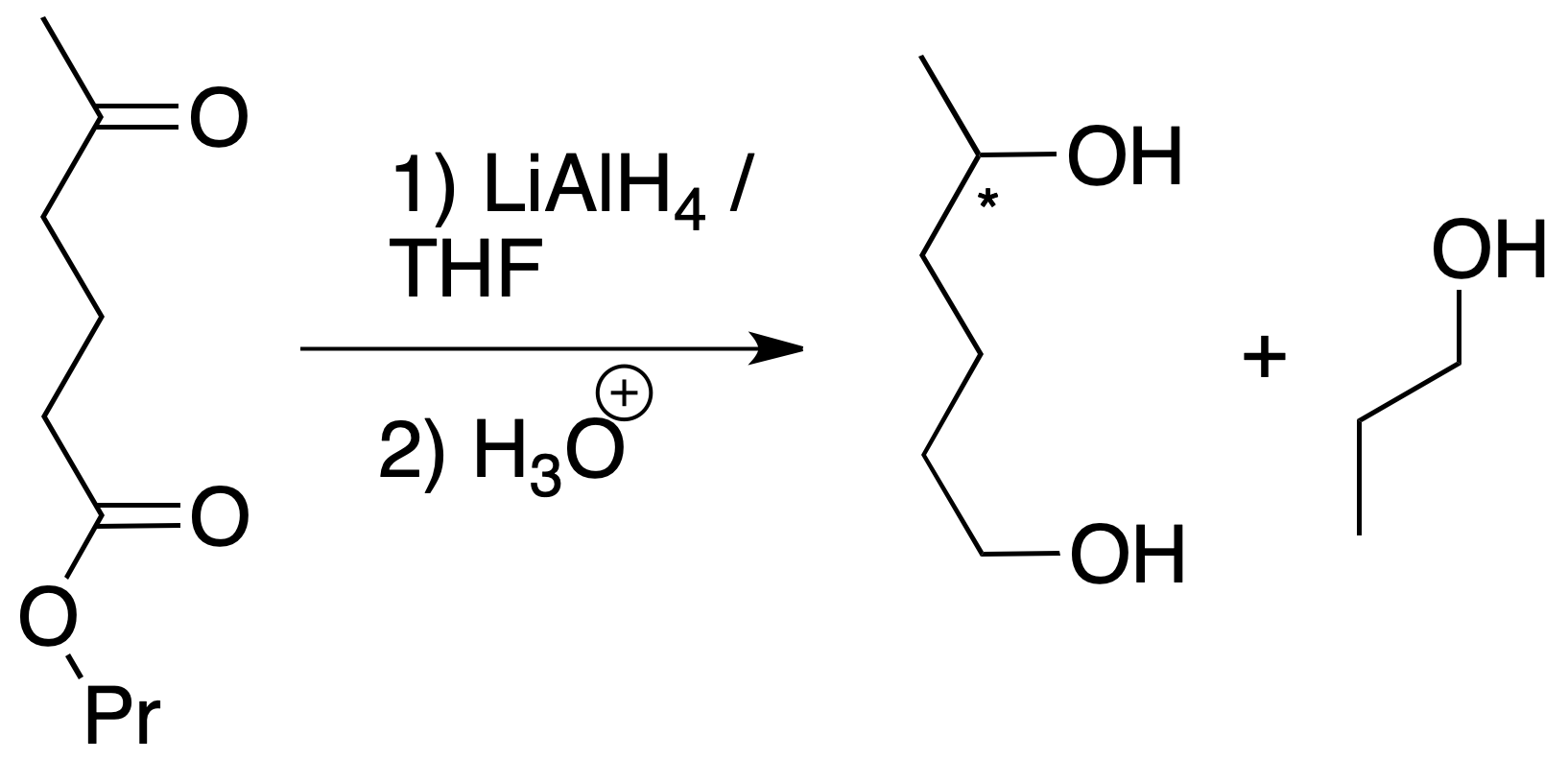
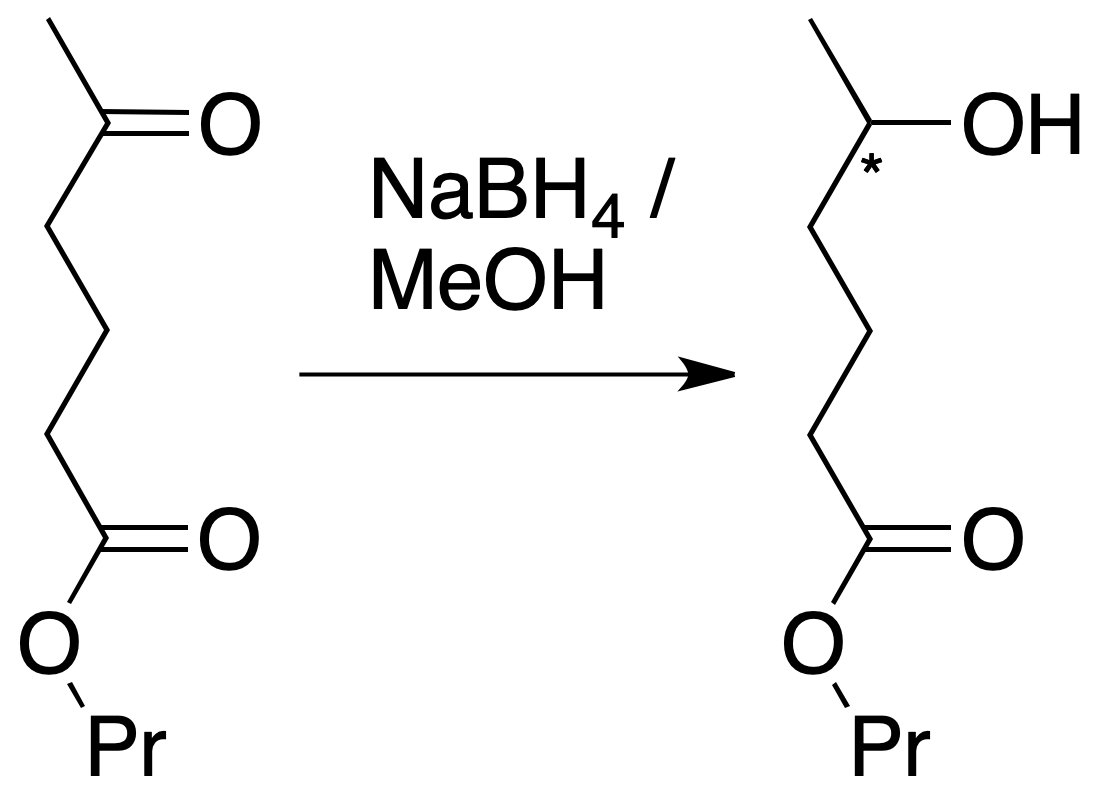
For the chosen example, the procedure to be followed would be as shown in the figure:
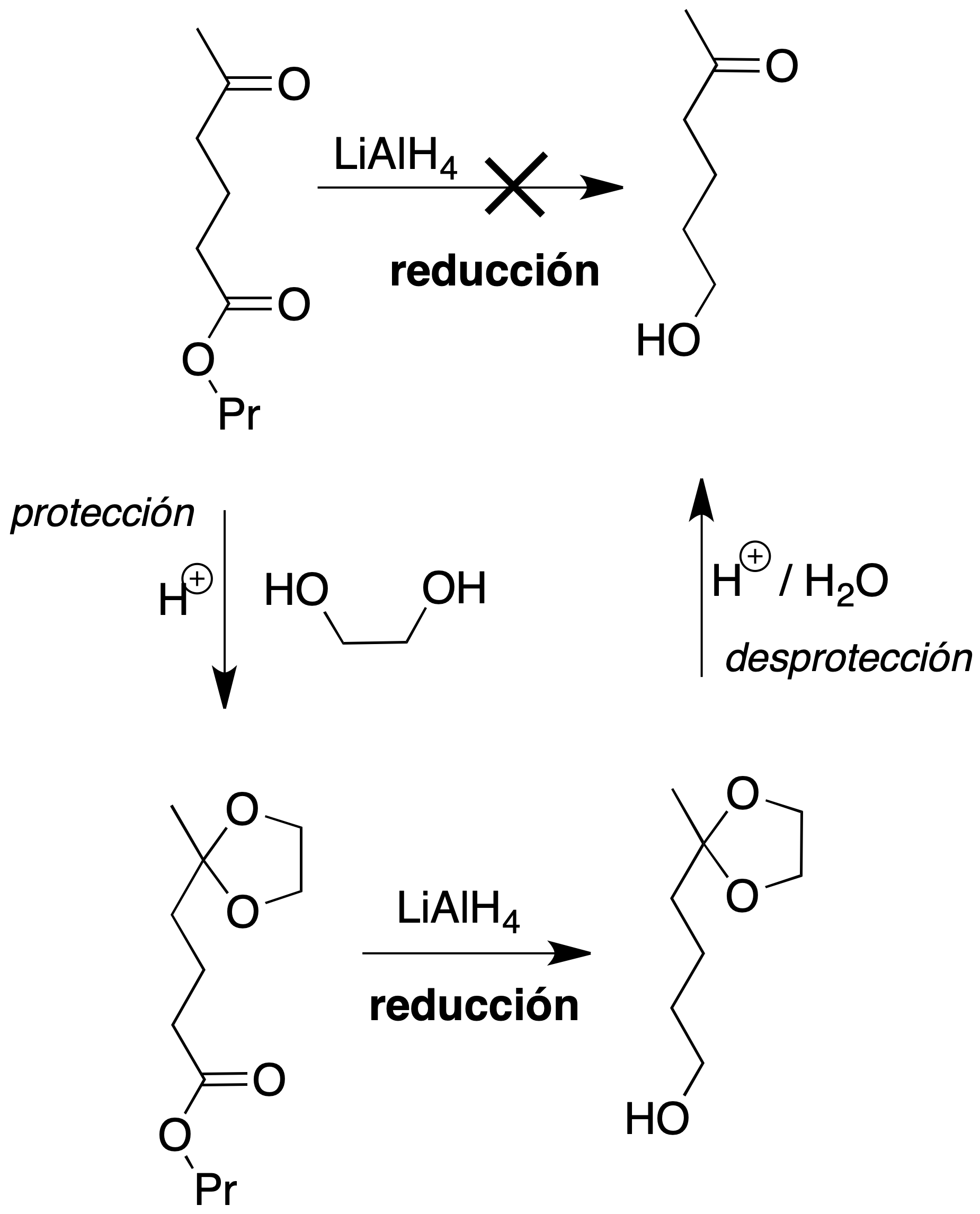
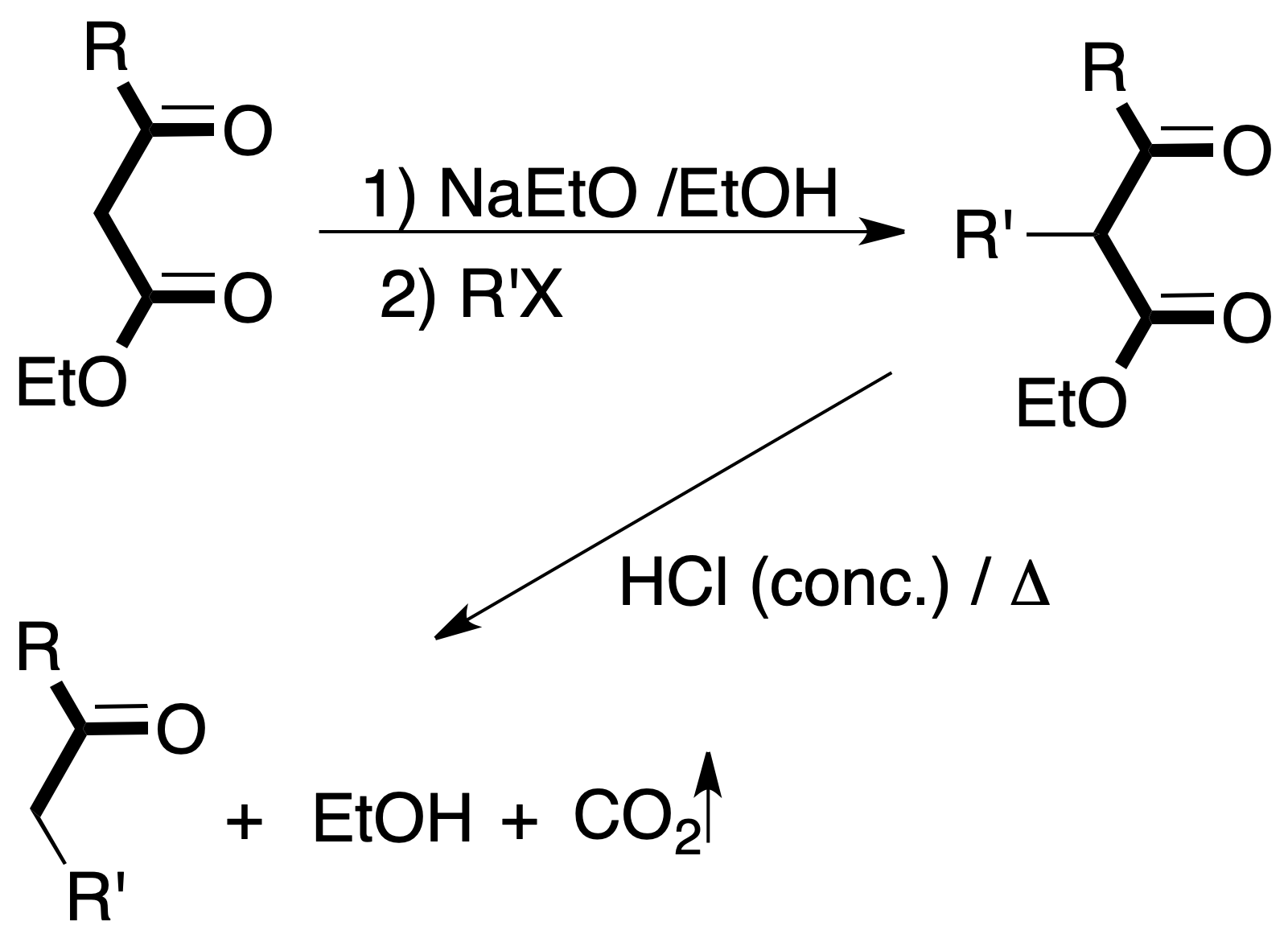
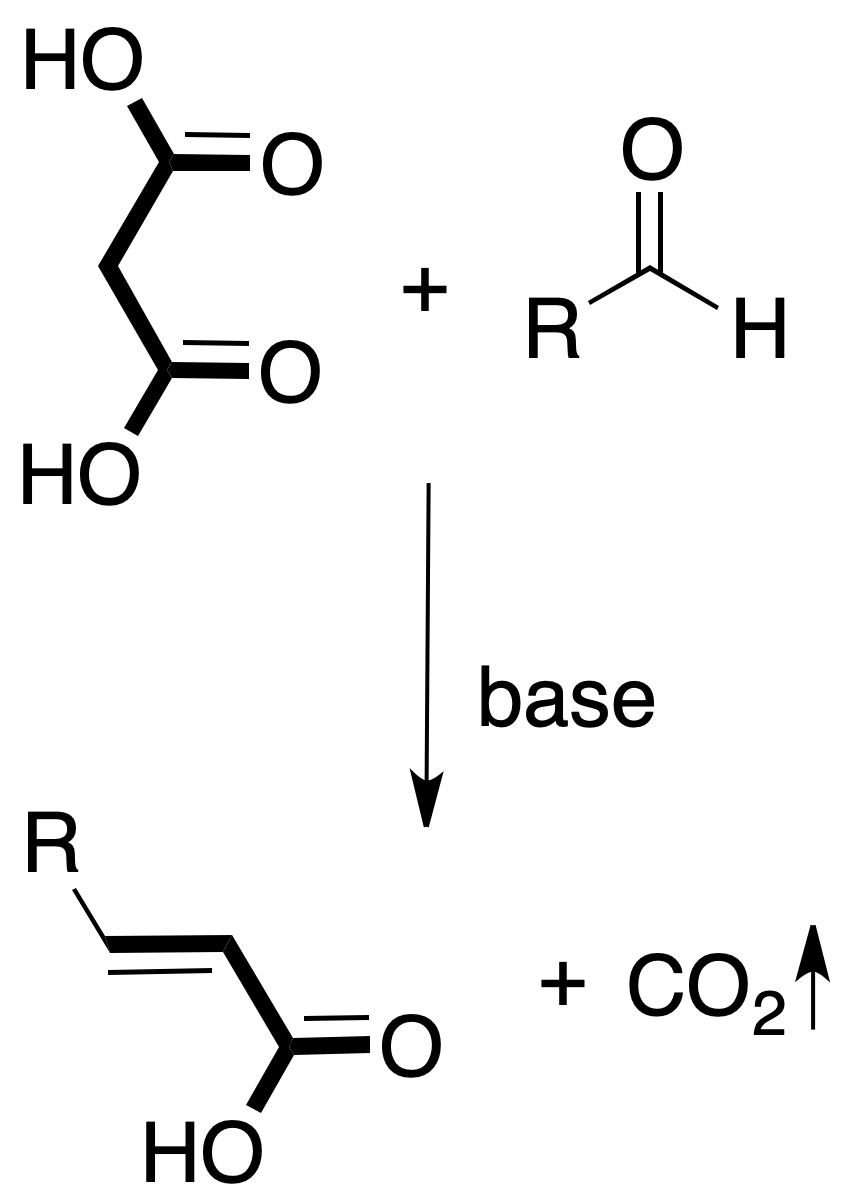
The reaction is carried out in the presence of a catalytic amount of base.
Table 1: Most common reductants in organic chemistry.
Aldehyde → alcohol 1º
Ketones → alcohol 2º
Generally does not reduce carboxylic acid, amide, and ester.
Ethanol, aqueous ethanol, diglime /
Carboxylic acid → alcohol 1º
Aldehyde → alcohol 1º
Ketone → alcohol 2º
Diethyl ether, THF /
Acyl chloride → aldehyde
Amide 3º → aldehyde
Aldehyde → alcohol 1° (slow)
Ketone → alcohol 2º (slow)
Azide, nitro derivative → amine.
Imine, nitrile → amine
Acyl chloride → aldehyde
Aldehyde → alcohol 1º
Ketones → alcohol 2º
Acid and ester → alcohol
Acyl chloride → alcohol
Azide, nitrile and amide → amine.
Halide and sulfonate → alkane
Diethyl ether, THF /
π-conjugated systems → miscellaneous compounds
Table 2: Most common oxidants in organic chemistry.
TransformationsSolvent /
Vicinal diol → carbonyl compound
Allyl and benzyl alcohol → carbonyl compound.
Alcohol 1º → aldehyde
Alcohol 2º → ketone
Oxidative alkene and alkyne cleavage
Aldehyde → carboxylic acid
Alcohol 2º → ketone
Alkene → vicinal diol
Alkyne → carboxylic acid
Pure water or mixtures with other solvents /
Collins reagent
Alcohol 1º → aldehyde
Alcohol 2º → ketone
Jones reagent
Alcohol 1º → carboxylic acid
Aldehyde → carboxylic acid
Alcohol 2º → ketone
Swern reagent
Alcohol 1º → aldehyde
Alcohol 2º → ketone
Vicinal diol → carbonyl compound
Alkene → vicinal diol
It is sometimes used in catalytic quantity accompanied by H2O2
The search for compounds with desired effects, with new functions and properties and the study of optimal methods for their synthesis are now the main objectives of the synthetic organic chemist. A large part of current organic synthesis is aimed at the preparation of compounds with biological activity.
The synthesis of taxol, erythromycins A and B, epothilones A and B, zaragocic acids, ecteinascidins, cephalostatins or vancomycin are some examples of syntheses that have been worked on for years.
Along with these syntheses, there has also been a spectacular development in the synthesis of oligosaccharides, peptides and compounds involved in micelle chemistry. All of them are important because of their participation in various biological processes. In recent years, progress has been made in the synthesis of oligosaccharides with the development of new glycosidation methods with efficient leaving groups that have given good yields and high stereoselectivity under mild conditions. New strategies based on orthogonal glycosidation reactions are recent examples. Peptides as protein segments can be recognized by enzymes or by the immune system and for this reason, many bioactive peptides have been prepared for pharmacological investigations and for clinical studies. However, the use of peptides as drugs is limited mainly due to their poor metabolic stability and problems of transport and assimilation. To remedy this disadvantage and to obtain pharmacologically useful compounds, modified peptides and peptide mimetics have been developed, some of which are promising anticancer agents. These peptides can intervene in complex biochemical and biological processes. It is hoped that with progress in the understanding of cellular processes it will be possible to selectively intervene or inhibit these processes using modified peptides and peptide-mimetics. These peptides can be obtained by design of their structure or by combinatorial chemistry approaches.
Another field of organic synthesis is the synthesis of medium-sized molecules in which two or more hydrophilic groups (acids, amino acids, alcohols, polyols, amines and amides, among others) are linked by a hydrophobic portion (long hydrocarbon chains, hardly branched and may include some unsaturation). These compounds known as bolaphiles have shown to be of great interest in micelle chemistry, and their potential in the preparation of ultrathin monolayer membranes and in their destruction.
Bolaphiles as dendritic macromolecules have also been of interest because of their ability to mimic enzymes, so they may act as therapeutic agents or tools to investigate molecular recognition and assembly.
It is easy to understand that in view of the great development of Organic Synthesis, it is very difficult to summarize. In view of the article by D. Seebach entitled “Organic Synthesis-where now?”, where after analyzing the most important achievements in the field of organic synthesis during the last years he announced “the primary focus of attention of all synthetic models will continue to shift towards catalytic and enantioselective variants; it will not be long before these modifications will be affordable with all standard reactions to convert achiral to chiral products“. According to this new synthetic methods will emerge in the fields of enantioselective synthesis and Organometallic Chemistry with special emphasis on catalytic methods.
Although there is in fact a practical division between what is Organic Synthesis and Inorganic Synthesis, such a division would certainly not stand up to a moderately rigorous conceptual argumentation. At the beginning of the 20th century, the interest of organic chemists focused solely on the generation of carbon-carbon bonds and on the use of molecules consisting only of carbon-hydrogen-oxygen-sulfur-nitrogen and some halogen. Alkali and alkaline earth metals only acted as counterions in salts and alcoholates. Many of the reactions of industrial application and preparative interest belong to this period, such as Claisen’s condensations, aldolica, Perkin’s condensation, Gries’ diazotization reaction, Michael’s conjugate addition, etc.
The later appearances of Friedel-Craft’s synthesis and Grignard’s organomagnesian reagents, on the one hand, and on the other, the heterogeneous hydrogenation of alkenes over nickel catalysts, accelerated the development of an organometallic chemistry that could complement the synthesis methods of organic chemists. However, its evolution was slow until transition metals made their appearance, as in the 1951 isolation of ferrocene by P. Pauson and T. Kealy. The carbon-metal bond acquired its prominence and has not abandoned it until today, when practically no metal is vetoed in organometallic chemistry.
However, in spite of the brilliant contributions to chemical synthesis coming from the main groups of the periodic table, for example hydroboration, the silica-aldol reaction or the radical reductions of tin hydrides, it seems that the prominence they boasted has been snatched away by the transition metals. The intrinsically different nature of the bonds formed by transition metals with organic ligands (18-electron rule, capto-dative coordination, etc.) and the also different mechanisms operating in these molecules (oxidative insertion, reductive elimination, catalytic cycles with successive expansion and contraction of the electronic sphere of the metal, etc.) mean that the organic ligands of the transition metals are not only the most important ones, but also the most important ones. ) mean that organic ligands bound to transition metals can undergo, for example, substitution reactions on sp 2 vinylic or aromatic carbon atoms and many other interesting transformations, difficult to achieve with the methods of traditional organic chemistry.
The demonstrated capacity of transition metals to generate catalytic cycles in which C-C bonds are formed has been the trigger for the so-called metallo-catalysis in its different forms: homogeneous, hetereogeneous and homogeneous-heterogeneous. The boom it has had, has been such that today it would be impossible to do without catalysis both in petrochemicals and in any industry that manufactures organic compounds. Therefore, it is convenient to make some considerations about the binomial metal-catalysis/Organic Synthesis that can later be important in the teaching of both, emphasizing the advantages of metal-catalyzed processes, namely: brevity, high chemo-, regio- and in some cases stereo-selectivity, low or null generation of by-products, mild reaction conditions and high productivity.
Another very important aspect of organometallic compounds is the so-called surface organometallic chemistry, which is closely related to the so-called homogeneous hetereogeneous catalysis, and refers to the study of the reactions of organometallic complexes with the surface of certain solid compounds such as metal oxides, zeolites and metals. It is, in fact, a new aspect of the Coordination Chemistry of organometallic compounds that can rival the catalysis performed by organometallic compounds both in homogeneous phase and in hetereogeneous conditions. This approach is called Organometallic surface chemistry. It has been commented that Synthetic Organometallic Chemistry can be the basis for developing the custom-made synthesis of catalysts in the sense that it can allow the meticulous preparation of the active center. However, and although there are certainly industrial applications of the technique, it appears that one of its limitations may be the scale-up of the reactions.
The analysis of the mental process followed to conceive the synthesis of a complex organic molecule can be as complicated as the synthesis itself. As we have already pointed out, we are all familiar with the classic debate held during the 1970s by Professors R.B. Woodward and E.J. Corey, as to whether synthesis is an art dependent on the genius and experience of the chemist or a logical activity that can be structured and systematized, even in the most difficult and complex cases. The consequences for teachers of these two approaches are very important, since the process of artistic creation cannot be transmitted to the student, while that of a 100% rational activity can. With the passage of time, it is the second option that is being consolidated and today the concepts of tuning, retron, disconnection, etc. are systematically included in the contents of the Organic Synthesis courses.
As a more sophisticated exponent of the above mentioned trend, the practice of research in Organic Synthesis counts on computer programs such as “CAMEO” (Computer Assisted Mechanistic Evaluation of Organic Reactions), “CASP” (Computer Assisted Synthesis Plan), “ORAC” (Organic Reaction Access by Computer), “SYNLIB” (Synthetic Library) or “CHAOS” (Computerization and Heuristic Applied to Organic Synthesis) etc. These combine affordability and ease of use with high degrees of reliability. Although now obsolete, one cannot fail to mention the original “LHASA” (Logic and Heuristic Applied to Synthetic Analysis) devised by Corey in the 1970s.
Organic Synthesis is one of the fields of Organic Chemistry that has evolved the most; the greater knowledge of Theoretical Chemistry, of reaction mechanisms on the one hand and the development of methods of isolation, purification and structural determination on the other have contributed to this; hence the most spectacular progress in Organic Synthesis began in the 1950s. A point of reference in the field of organic synthesis is the synthesis of strychnine described by R.B. Woodward in 1954, who received the Nobel Prize in 1965 for his achievements in the field of organic synthesis.
The synthesis of strychnine by Woodward can be considered a milestone in the development of modern Organic Synthesis, both for the design and execution of the synthesis of such a complex molecule. The second total synthesis of strychnine was performed by P. Magnus in 1992. In 1993, L.E. Overman published the first and so far the only enantioselective synthesis.
Another milestone in Organic Synthesis was marked by E.J. Corey (Nobel Prize in 1990), who systematically applied new principles and methodologies in the design of syntheses. As he himself analyzes, during the first half of the 20th century, organic chemistry focused its attention on understanding the carbon structure and transformations of organic compounds. Chemical changes were looked at in the direction of the chemical reaction, i.e. from reactants to products. Most of the syntheses developed were carried out by selecting the appropriate starting material and investigating a series of reactions that transformed the material into the desired product. In the mid 1960’s a different and more systematic approach was developed, which consisted of structurally analyzing the reaction product and manipulating the structure in reverse. This is known as retrosynthetic analysis and was developed by E.J. Corey. This author was the forerunner of today’s computer synthesis design. These two fathers of Organic Synthesis, Woodward and Corey, conceive it as an art or as a science. Professor F. Serratosa summarizes it as follows: “for R.B. Woodward, organic synthesis is a source of emotion, provocation and adventure and can also be a noble art; for E.J. Corey, organic synthesis is an essentially logical and rational activity”. E.J. Corey himself indicated this in his book “The logic of Chemical Synthesis”.
Since these authors, organic synthesis has undergone such a spectacular advance that it is impossible to list the syntheses described and the researchers who have contributed to it. The boom in organic synthesis since the 1960s has been so great that thousands of compounds with complex structures have been synthesized before the beginning of the 1990s. The complexity of the synthesized systems seems to culminate with the synthesis of palytoxin by Y. Kishi in 1987, the most potent natural toxin known with 64 stereocenters and 7 double bonds for which 2 71 stereoisomers are possible.
The great advances in organic synthesis have made some authors wonder whether it has reached its limits as a science, since nowadays it can be considered that the synthesis of any structure, however complex it may be, can be carried out if there is access to knowledge, if imagination is used and if sufficient economic support is available. On the other hand, some of the classic objectives addressed in the synthesis of a compound, such as the confirmation of the structure of a natural product, the theoretical interest or the intellectual challenge to achieve a total synthesis, seem to have lost some validity.